Chemistry 3
Organic chemistry: Precipitaion, combustion, decomposition, corrosion, displacement reactions (and all the fun stuff from last year)
Table of Contents
- Primers
- Precipitation reactions
- Combustion reactions
- Decomposition
- Corrosion
- Displacement reaction
- wow what’s that? they released the study guide?
- Chemistry
Hey there! Please be advised that these notes are grouped in concepts, not textbook chapters. Feel free to use the textbook, or use that neat little search bar up the top there! Thanks!
Primers
Polyatomic ion valencies you need to remember
| Valency | Ion | Formula |
|---|---|---|
1+ | Ammonium | NH₄ |
1- | Hydroxide | OH |
1- | Nitrate | NO₃ |
2- | Carbonate | CO₃ |
2- | Sulfate | SO₄ |
3- | Phosphate | PO₄ |
Remember! All transition metals have a valency of 2+ except for Gold, which has a valency of 1+
Endothermic and exothermic reactions
- Endothermic reactions absorb heat
- respiration
- metal + acid
- heat packs and warmers
- Exothermic reactions release heat
- photosynthesis
- ice packs
TIP! To remember the difference between endothermic and exothermic reactions - remember that endo sounds like inhale (take in), and exo sounds like exhale (release) with respect to heat.
Hydrocarbons
Hydrocarbons are compounds made up of only hydrogen and carbon.
| **Molecular Formula ** | Name |
|---|---|
| CH4 | methane |
| C2H6 | ethane |
| C4H8 | buthane |
| C3H8 | propane |
| C4H10 | butane |
| C5H12 | pentane |
| C6H14 | hexane |
| C7H16 | heptane |
| C8H18 | octane |
| C9H20 | nonane |
| C10H22 | decane |
Source: Table 16 at Introductory Chemistry, Chapter 16.1
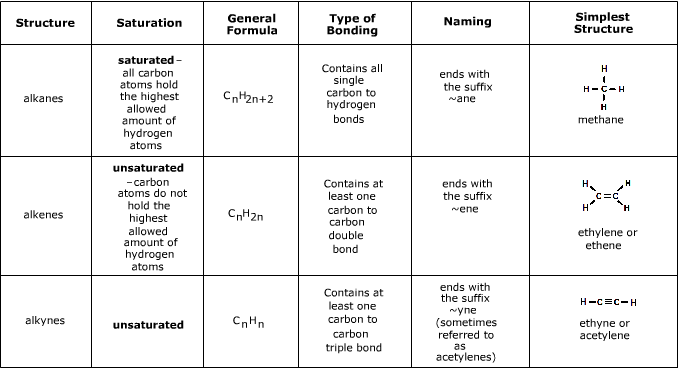
- Your standard hydrocarbons are known as alkanes (methane
CH4, ethaneC2H6, buthaneC4H8, etc) - they are saturated and are joined by single bonds.- They are relatively unreactive apart from combustion reactions
- Alkanels or *alcohols (methanol
CH3OH, ethanolC2H5OH, butanolC4H7OH, etc) contain saturated hybocarbons bound to a hydroxide-OH.
Reactivity series

Metals that are higher up the reactivity series are more reactive and more vulnerable to reactions such as corrosion.
Precipitation reactions
When solutions of different ionic compounds react with each other, they sometimes produce a precipitate - insoluble solid.
For example:
silver nitrate + sodium chloride solution -> sodium nitrate + silver chloride (precipitate)
Precipitation reactions are predicted using a table of solubilities.
| Anion | All soluble/insoluble | except | (slightly) |
|---|---|---|---|
Chlorides Cl- | all soluble except | Ag+ • Hg₂2+ | Pb2+ |
Bromides Br- | all soluble except | Ag+ • Hg₂2+ • Hg2+ | Pb2+ |
Iodides I- | all soluble except | Ag+ • Hg₂2+ • Hg2+ • Pb2+ | |
Nitrates NO₃- | all soluble, no exceptions | ||
Sulfates SO₄2- | all soluble except | Sr2+ • Ba2+ • Hg₂2+ • Hg2+ | Ca2+ • Ag+ |
Phosphates PO₄3- | all insoluble except | Na+ • K+ • Rb+ • Cs+ • NH₄ | |
Carbonates CO₃2- | all insoluble except | Na+ • K+ • Rb+ • Cs+ • NH₄ | |
Hydroxides OH- | all insoluble except | Li+ • Na+ • K+ • Rb+ • Cs+ • Ba2+ • NH₄ | Ca2+ • Sr2+ |
Cool tip! Given two ionic compounds (solutions) swap the metals OR swap the ions. For example, given:
sodium hydroxide + lead nitrate
# swap the metals (or gases) around!
-> lead hydroxide + sodium nitrate
Sometimes there will be no precipitate formed. Therefore, check your table of solubilities to see whether a precipitate can be formed.
Combustion reactions
Combustion reactions occur when oxygen reacts with another substance to produce energy in the form of heat, light and sound.
Alkanes and alkenes on BBC Bitesize (GCSE)
A combustion reaction is an exothermic reaction as it releases heat, and it often involves hydrocarbons.
Complete combustion
In complete combustion all reactants will combust provided sufficient oxygen is available. Carbon oxidises to carbon dioxide, and hydrogen oxides to water.
Remember! Oxidation is the process of gaining oxygen.
The general equation for complete combustion is:
hydrocarbon + oxygen -> carbon dioxide + water
For example, in methane:
methane + oxygen -> carbon dioxide + water
CH4 + O -> CO2 + H2O
Simples!
Incomplete combustion
When there isn’t enough oxygen to allow for a complete combustion reaction, incomplete combustion occurs instead.
The general equation for incomplete combustion is:
hydrocarbon + oxygen -> carbon monoxide + carbon + water
For example, in methane (balanced chemical eq.):
methane + oxygen -> carbon monoxide + carbon + water
4CH4 + 5O2 -> 2CO + 2C + 8H2O
Note that carbon dioxide has been replaced with carbon monoxide + carbon
- Carbon monoxide is poisonous!
- Carbon is produced in the form of soot.
It was determined in an experiment that less energy is released with less carbon atoms.
Decomposition
i swear we never learnt this
Decomposition occurs when one substance breaks down into two substances. It is an example of an endothermic reaction
The general equation for a decomp. reaction is:
AB -> A + B
For example, with calcium carbonate1
CuCO3 -> CuO + CO2
It is usually observable by colour change.
Corrosion
Corrosion occurs when metal is exposed to air, water or other substances.
Corrosion on BBC Bitesize (National 4)
Some materials are more vulnerable to corrosion than others
- Iron and steel rust
- Tin doesn’t rust
In the real world (yea with all this coronavirus stuff I guess noone knows what that is anymore eh) corrosion is prevented through different measures:
- Painting/greasing: Paint or grease protects the metal from air, water, and sunlight.
- Electro-plating: tin-plated iron won’t rust as the iron is not exposed, and tin doesn’t rust. Electro-plating involves covering a metal in a thin layer of a less reactive metal.
- Sacrificial Protection: Magnesium is more reactive in corrosion, therefore it can be sacrificed instead of the material under it. Sacrificial protection involves coating a metal in a more reactive metal.
- Galvanising is similar to sacrificial protection, but it specifically refers to coating iron or steel with a zinc protective layer.
Displacement reaction
In a displacement reaction, a more reactive metal will displace a less reactive metal from its compounds2
Displacement reactions on BBC Bitesize (KS3)

Some metals are more reactive than others.
Fe + Pb(NO₃)₂ -> Fe(NO₃)₂ + Pb # displacement has occured
Mg + CuSO -> MgSO₄ + Cu # displacement has occured
Pb + Fe(NO₃)₂ -> NO REACTION # no displacement
Au + Pb(NO₃)₂ -> NO REACTION # no displacement
According to the Reactivity series, Magnesium is more reactive than zinc, and zinc is more reactive than iron, and iron is more reactive than copper, etc. This is best represented in this table displaying different reactions and whether an observable and visible reaction occurs.
| Magnesium | Zinc | Iron | Copper | |
|---|---|---|---|---|
| Magnesium sulfate | ✘ | ✘ | ✘ | ✘ |
| Zinc sulfate | ✔ | ✘ | ✘ | ✘ |
| Iron sulfate | ✔ | ✔ | ✘ | ✘ |
| Copper sulfate | ✔ | ✔ | ✔ | ✘ |
| Reactions seen | 3 | 2 | 1 | 0 |
Source: BBC Bitesize ofc!
wow what’s that? they released the study guide?
Biology
Chemistry
- Describe the indications that a chemical reaction has occurred
- Group compounds into ionic and covalent depending on their properties
- Explain the Law of Conservation of Mass
- Describe and write equations for combustion, decomposition and precipitation reactions
- Write word and balanced chemical equations for the following reactions
- metal and acid reactions (also describe the test for hydrogen)
- metal carbonate and acid reactions (also describe the test for carbon dioxide)
- decomposition reactions involving metal carbonates and metal oxides
- Use the solubility rules (provided – no need to memorise) to predict whether reactions will produce a precipitate
Chemistry
Describe the indications that a chemical reaction has occurred
To quote the Minnesota Science Teachers Education Project…
There are five signs of a chemical change:
- Color Change
- Production of an odor
- Change of Temperature
- Evolution of a gas (formation of bubbles)
- Precipitate (formation of a solid)
In a precipitation reaction, this might involve the formation of a solid (precipitate). In a combustion reaction, a change of temperature or production of a gas. In a corrosion reaction, this might involve a change of temperature or a precipitate (rust). In a displacement reaction, this might involve a colour change of sorts.
Group compounds into ionic and covalent depending on their properties3
Ionic compounds:
- have higher melting points and electrical conductivity
- electrostatic bonds between ions
- tend to be hard and brittle
- solid at room temp.
- occurs between a metal and a non-metal
Covalent compounds:
- have lower melting points and electrical conductivity
- share electrons
- tend to be soft and flexible
- liquid/gaseous at room temp.
- occurs between two non-metals
Explain the Law of Conservation of Mass
The law of conservation of mass or principle of mass conservation states that for any system closed to all transfers of matter and energy, the mass of the system must remain constant over time, as the system’s mass cannot change, so quantity can neither be added nor be removed. Therefore, the quantity of mass is conserved over time.4
In a nutshell:
mass cannot be created nor destroyed
Describe and write equations for combustion, decomposition and precipitation reactions
sigh
Combustion
Complete combustion
hydrocarbon + oxygen -> carbon dioxide + water
Incomplete combustion
hydrocarbon + oxygen -> carbon monoxide + carbon + water
Decomposition
A-B -> A + B
Precipitation
A-B + C-D -> A-D + B-C
where A and C are metals
and B and D are non-metals
It might also help knowing your other general equations:
Combination
A + B -> A-B
Corrosion
metal + oxygen gas -> metal oxide
Acid + Base
acid + base -> salt + water
Acid + Carbonate
acid + carbonate -> salt + water + carbon dioxide
Acid + Metal
acid + metal -> salt + hydrogen
Use the solubility rules (provided – no need to memorise) to predict whether reactions will produce a precipitate
Something is soluble if it can dissolve in water. In an exam, you’ll be given a solubility rules sheet, and you’ll need to use it (in conjunction with the periodic table)
-
https://www.bbc.co.uk/bitesize/guides/zqd2mp3/revision/5 ↩
-
Displacement reactions on BBC Bitesize (KS3) - https://www.bbc.co.uk/bitesize/guides/zqwmxnb/revision/3 ↩
-
Wikipedia, the free encyclopedia. https://en.wikipedia.org/wiki/Conservation_of_mass ↩